Have you ever wondered why a balloon expands when you blow air into it, or why ice melts into liquid water? The answers to these seemingly simple questions lie within the fascinating realm of the kinetic molecular theory, a fundamental concept in chemistry that helps us understand the behavior of matter at the molecular level.
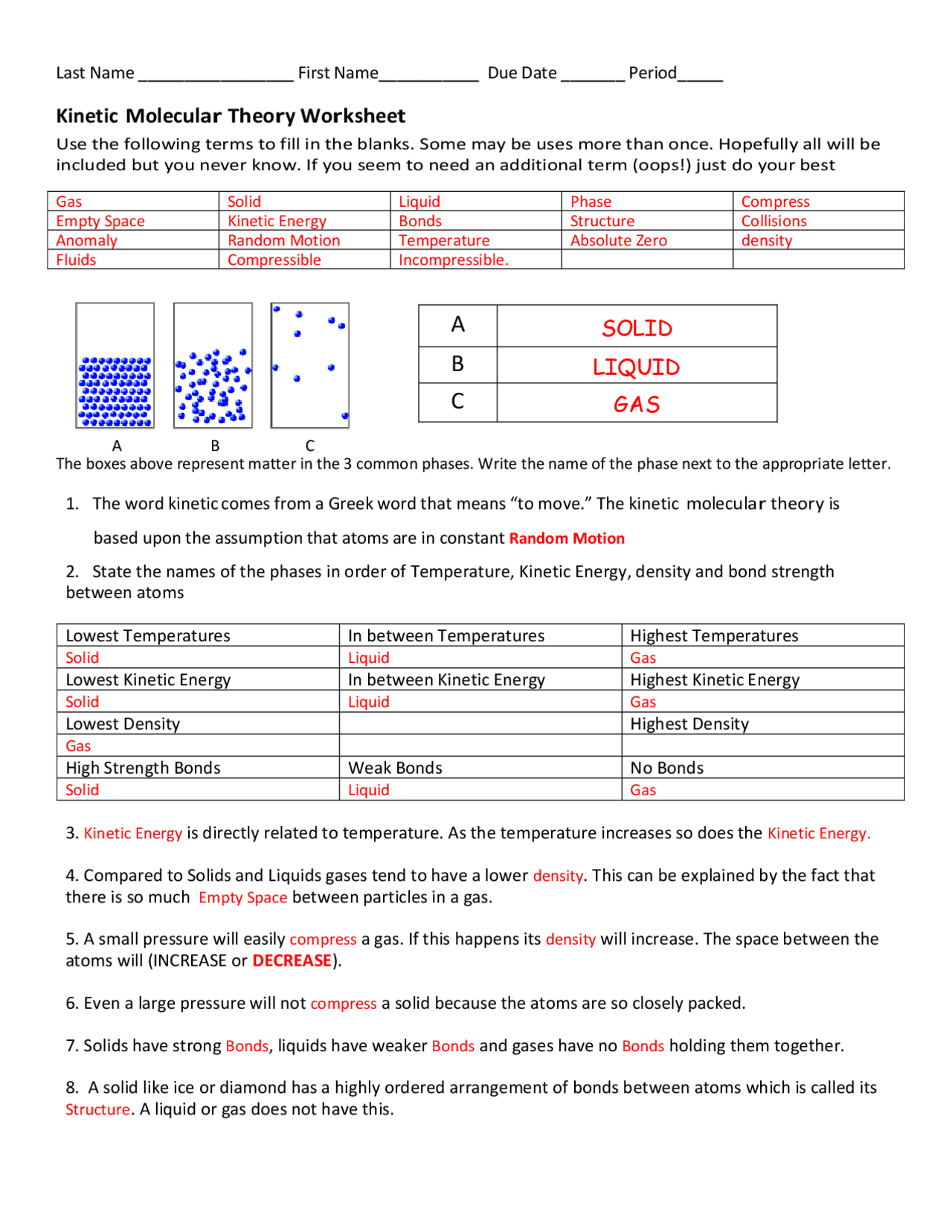
Image: www.docsity.com
This article delves into the intricacies of the kinetic molecular theory, exploring its key postulates, applications, and how to navigate the POGIL (Process Oriented Guided Inquiry Learning) activities designed to deepen your understanding. We’ll break down the theory into manageable chunks, addressing common misconceptions and providing practical examples to make it easier to grasp.
The Building Blocks of the Kinetic Molecular Theory
The kinetic molecular theory is a model that explains the behavior of gases, liquids, and solids based on the idea that all matter is composed of tiny particles in constant motion. It offers a powerful framework for understanding phenomena like diffusion, pressure, temperature, and phase changes. Let’s explore the key postulates that form the foundation of this theory:
1. Matter is composed of particles in constant motion.
Imagine tiny, energetic particles constantly bouncing around – this is the essence of the first postulate. These particles can be atoms, molecules, or ions, depending on the substance. The kinetic molecular theory emphasizes that these particles are never truly stationary, even in solids.
2. The average kinetic energy of particles is directly proportional to temperature.
The hotter a substance gets, the faster its particles move. This relationship between temperature and kinetic energy is fundamental to understanding gas behavior. As temperature increases, particles collide more frequently and with greater force, leading to increased pressure.

Image: worksheetsdynamic1.blogspot.com
3. Particles interact through collisions.
These interactions aren’t just random events; they play a crucial role in defining the properties of matter. Collisions between particles transfer energy, influencing the motion and energy distribution of the entire system.
4. The volume occupied by the particles themselves is negligible compared to the total volume of the gas.
In gases, particles are spaced far apart, and their individual volumes are considered insignificant compared to the overall space they occupy. This explains the compressibility of gases – they can be squeezed into a smaller volume because particles move freely and have ample space between them.
5. The average kinetic energy of the particles is independent of their masses.
This means that lighter particles, like hydrogen gas molecules, move faster than heavier particles, like oxygen gas molecules, at the same temperature. This difference in speed has significant implications for diffusion and other properties.
POGIL: A Hands-On Approach to Learning
POGIL activities are designed to encourage active learning and promote deeper understanding of scientific concepts. These guided inquiry exercises challenge you to explore and analyze data, apply concepts to real-world scenarios, and draw conclusions based on your observations. POGIL activities are valuable tools for mastering the kinetic molecular theory because they allow you to:
-
-
* **Active learning**: Instead of passively absorbing information, POGIL activities engage you in the learning process.
* **Collaborative learning**: Working in groups, you can share ideas, brainstorm solutions, and learn from each other.
* **Problem-solving**: POGIL problems often present real-world scenarios that require applying the theory to solve practical issues.
* **Critical thinking**: You develop the ability to analyze data, draw inferences, and support your arguments with evidence.
Decoding the POGIL Kinetic Molecular Theory Answer Key
The POGIL answer key is a valuable resource that can help you verify your work, identify areas where you need further clarification, and deepen your understanding of the theory. It’s not meant to be a shortcut to the answers; rather, it serves as a guide to ensure you’re on the right track. Here are some tips for effectively using the answer key:
- Focus on the process: Don’t simply copy the answers from the key. Instead, use it to check your logic, reasoning, and conclusions.
- Understand the explanations: Pay attention to the explanations provided with the answers. These explanations reveal the underlying concepts behind each question and solution.
- Identify areas of weakness: If you consistently find yourself struggling with certain types of questions, it’s a sign you need to revisit those concepts.
- Practice, practice, practice: Working through additional problems and applying the theory to different scenarios will further solidify your understanding.
Applications of the Kinetic Molecular Theory
The kinetic molecular theory isn’t just an abstract concept; it has widespread practical applications in fields ranging from chemistry to engineering. Here are some examples:
- Chemical reactions: The theory helps us understand how temperature and pressure affect reaction rates, allowing us to optimize industrial processes.
- Gas dynamics: It forms the basis for calculating gas pressure, volume, and temperature, which are crucial concepts in engineering and atmospheric science.
- Phase changes: The theory explains the transitions between solid, liquid, and gas states, providing insights into melting, freezing, boiling, and condensation.
Beyond the Basics: Exploring Current Research
The kinetic molecular theory continues to evolve as scientists uncover new insights into the behavior of matter at the molecular level. Current research focuses on areas like:
- Nanomaterials: Understanding the behavior of particles at the nanoscale, where quantum effects become significant.
- Plasma physics: Investigating the properties and applications of ionized gases, which exhibit unique behavior compared to ordinary gases.
- Computational modeling: Developing sophisticated simulations to predict the behavior of complex molecular systems.
Pogil Kinetic Molecular Theory Answer Key
Conclusion:
The kinetic molecular theory empowers us to understand and predict the behavior of matter at the atomic and molecular level. By mastering its postulates, we gain a fundamental framework for explaining a wide range of phenomena, from gas behavior and phase changes to the intricacies of chemical reactions. POGIL activities provide an engaging and interactive way to learn about this theory, and the answer key can be a valuable tool for ensuring you’re on the right path. But remember, the true value comes from actively engaging with the material, exploring its applications, and delving deeper into the cutting-edge research that continues to shape our understanding of matter.





