Have you ever wondered why your stomach churns after a spicy meal or why soap feels slippery? The answers lie within the fascinating world of acids and bases – two fundamental concepts in chemistry that govern countless aspects of our lives. From the acidic tang of lemon juice to the alkaline properties of baking soda, these chemical entities play a crucial role in our everyday experiences. But understanding their complexities can feel daunting. That’s where the power of POGIL, or Process Oriented Guided Inquiry Learning, comes in. This innovative approach, embraced by educators worldwide, empowers students to become active participants in their learning journey, unlocking the secrets of acids and bases through hands-on exploration and critical thinking.

Image: www.pinterest.com
In this journey of exploration, we’ll delve into the fundamentals of acids and bases, unraveling their definitions, properties, and reactions. We’ll uncover the intriguing history of these concepts, from ancient alchemists to modern advancements, discovering how our understanding has evolved over time. By engaging with POGIL exercises, we’ll gain invaluable insights into the practical applications of acid-base chemistry, from the production of essential pharmaceuticals to the design of sustainable energy solutions. As we progress, you’ll discover how this knowledge can empower you to make informed decisions about the world around you.
Defining the Fundamentals of Acids and Bases
At the heart of acid-base chemistry lie two fundamental concepts: the Brønsted-Lowry Theory and the Lewis Theory. The Brønsted-Lowry Theory, proposed by Johannes Nicolaus Brønsted and Thomas Martin Lowry, defines acids as proton donors and bases as proton acceptors. This model simplifies the understanding of acid-base reactions, explaining how substances exchange hydrogen ions (protons) with each other. For instance, when hydrochloric acid (HCl) dissolves in water, it donates a proton to water, forming hydronium ions (H3O+) and chloride ions (Cl-). Conversely, a base like sodium hydroxide (NaOH) readily accepts protons, forming hydroxide ions (OH-) and sodium ions (Na+).
The Lewis Theory, developed by Gilbert Newton Lewis, offers a broader perspective. It defines acids as electron-pair acceptors and bases as electron-pair donors. This perspective encompasses a wider range of chemical reactions, including those involving coordination complexes, where a central metal atom accepts electron pairs from surrounding ligands (molecules or ions). The Lewis theory emphasizes the role of electron transfer in acid-base reactions, providing a more comprehensive understanding of chemical bonding.
The pH Scale: A Measure of Acidity and Basicity
To quantify the acidity or basicity of a solution, we rely on the pH scale, ranging from 0 to 14. A pH value of 7 indicates neutrality, where the concentrations of hydrogen and hydroxide ions are equal. Solutions with pH values below 7 are acidic, with higher concentrations of hydrogen ions, while solutions with pH values above 7 are basic, with higher concentrations of hydroxide ions. The pH scale is logarithmic, meaning each unit represents a tenfold change in hydrogen ion concentration. This logarithmic nature allows for a convenient way to express a wide range of acidity or basicity.
For example, a solution with a pH of 2 is ten times more acidic than a solution with a pH of 3, and one hundred times more acidic than a solution with a pH of 4. This scale is crucial in understanding the behavior of various chemical reactions, monitoring environmental conditions, and maintaining the health of biological systems.
POGIL: Unleashing the Power of Active Learning
POGIL provides a transformative learning experience by shifting the focus from passive absorption to active engagement. Instead of simply listening to lectures or reading textbooks, students become active participants in the learning process. They collaborate in teams, guided by inquiry-based questions, to develop a deep understanding of the concepts. The POGIL approach encourages them to critically analyze information, propose solutions, and debate their findings, fostering a deeper understanding of the subject matter.
When applied to acid-base chemistry, POGIL exercises offer a practical way to explore the concepts through real-world examples. They challenge students to analyze chemical reactions, predict the pH of solutions, and design experiments to test their hypotheses. By actively engaging with the material, they gain a deeper understanding of the fundamental principles and their practical significance.
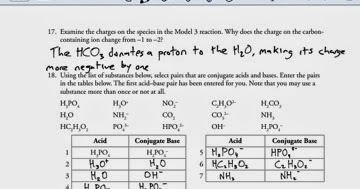
Image: jreitzchem.blogspot.com
Examples of POGIL Exercises in Acid-base Chemistry
POGIL exercises in acid-base chemistry can encompass a wide range of topics, providing students with opportunities to explore various applications of the concepts. Here are a few examples:
-
Identifying Acids and Bases: Students can be presented with a list of chemical compounds and tasked with identifying which are acids and bases based on the Brønsted-Lowry theory. This exercise encourages them to apply the definition and practice recognizing proton donors and acceptors.
-
Predicting pH: Students can given a series of solutions and asked to predict their pH based on the known properties of acids and bases. This exercise deepens their understanding of the pH scale and its relationship to the concentration of hydrogen and hydroxide ions.
-
Designing Experiments: Students can be challenged to design experiments to test the pH of common household substances, like lemon juice or baking soda, using pH indicators. This activity provides hands-on experience and encourages them to apply their knowledge to real-world scenarios.
-
Exploring Acid-Base Titration: Students can learn about the concept of titration, a method used to determine the concentration of an unknown solution through controlled reactions with a solution of known concentration. This exercise demonstrates the practical application of acid-base reactions in analytical chemistry and its significance in various industries.
Unveiling the Applications of Acid-base Chemistry
Acid-base chemistry has widespread applications, shaping our world in countless ways. Here are a few examples that highlight its significance:
-
Food Production: Acids are essential for flavoring food, preserving ingredients, and creating culinary masterpieces. From the tartness of citrus fruits to the acidity of vinegars, these compounds add complexity and depth to our food experiences.
-
Medicine: Acids and bases play critical roles in various medical applications. Antacids, often used to neutralize excess stomach acid, are examples of bases that relieve heartburn and indigestion. Acidic solutions are also used in the manufacturing of pharmaceuticals, and base solutions are used for disinfecting wounds and sterilizing medical instruments.
-
Household Products: Acids and bases are ubiquitous in our homes, contributing to the cleanliness and functionality of our surroundings. Cleaning agents, like vinegar and bleach, utilize acids and bases to remove dirt, grime, and microorganisms, keeping our homes sanitized.
-
Environmental Protection: Acid-base chemistry is essential for monitoring and mitigating environmental pollution. The pH of water bodies is a vital indicator of their health, and imbalances can have detrimental effects on aquatic ecosystems. Understanding acid-base reactions is crucial for developing strategies to address environmental challenges.
Expert Insights: Navigating the World of Acids and Bases
Dr. Emily Carter, a renowned chemist and educator, underscores the importance of understanding acid-base chemistry in today’s world. “As we delve deeper into scientific advancements, the principles of acid-base chemistry continue to play a crucial role,” she explains. “From the development of sustainable energy technologies to the design of new materials, our understanding of these concepts empowers us to address complex challenges facing our society.”
Dr. Carter further emphasizes the transformative power of active learning methods like POGIL. “By engaging students in a hands-on, collaborative learning experience, we foster their critical thinking skills, problem-solving abilities, and scientific curiosity. This active approach empowers them to become not just passive learners but active participants in the creation of knowledge,” she adds.
Acid And Bases Pogil Answer Key
The Power of Exploration: Embracing a World of Discovery
As we’ve journeyed through the world of acids and bases, we’ve uncovered their profound significance, from the everyday experiences that shape our lives to the groundbreaking scientific discoveries that advance our understanding of the world. POGIL’s emphasis on active learning empowers us to unravel these complexities through hands-on exploration, critical thinking, and collaborative learning. Embrace this approach, embrace the power of inquiry, and your journey into the world of acids and bases will be filled with exciting discoveries.
Now, it’s your turn to explore further. Take a closer look at the reactions happening around you, from the fizzing of soda to the rusting of metal. Ask questions, investigate the unknown, and you’ll be amazed by the wonders of acid-base chemistry that await your exploration. The world of scientific discovery is vast, and POGIL is your guide. Embrace the journey, and the knowledge you gain will empower you to make informed decisions about the world around you.





