Have you ever wondered how scientists create new materials, understand the reactions happening within our bodies, or develop new medicines? The answer lies in the intricate world of chemistry, where chemical reactions play a pivotal role. At the heart of this world lies the concept of balancing chemical equations, a fundamental skill that unlocks the understanding of how atoms rearrange themselves during chemical transformations.
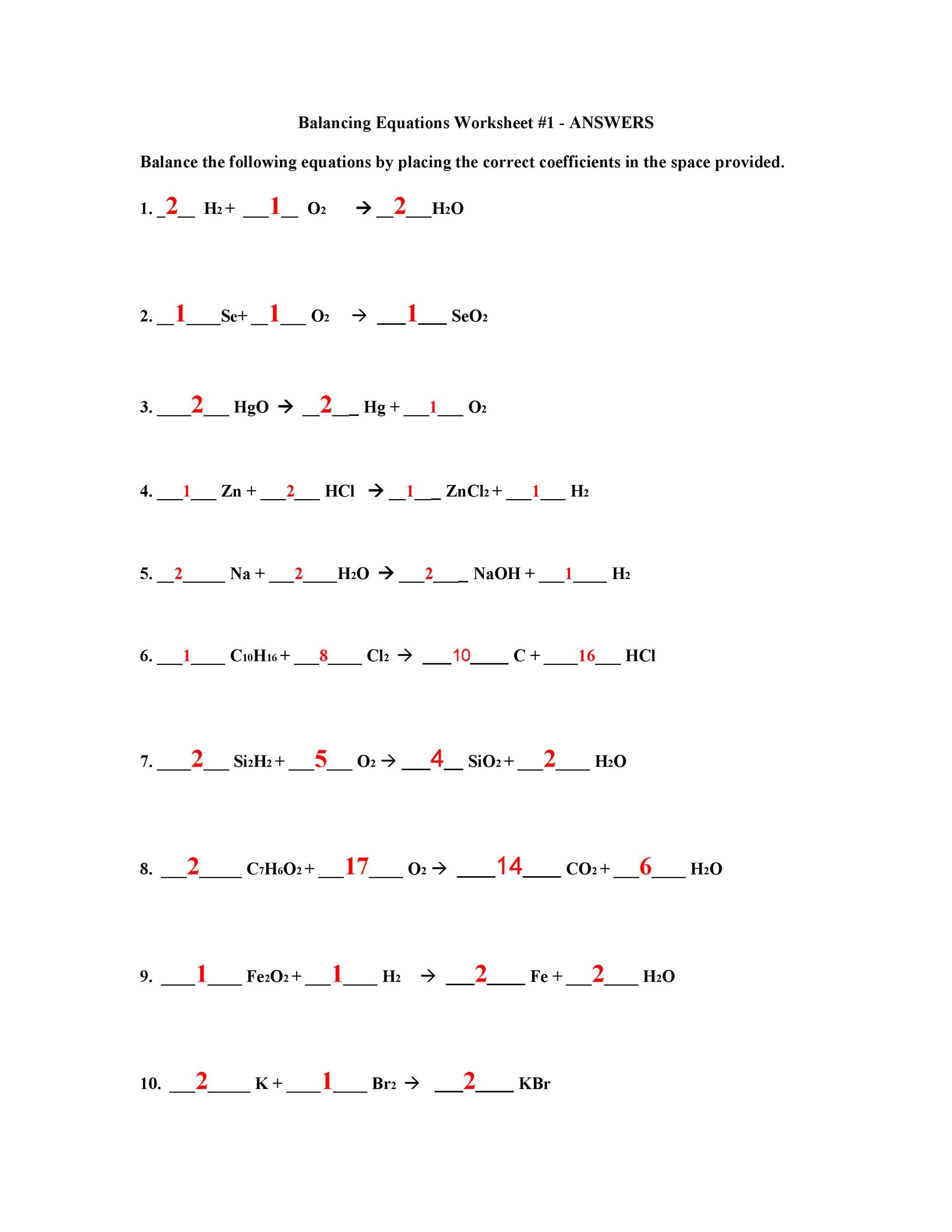
Image: templatelab.com
Imagine trying to cook a delicious meal without following a recipe. The results, if any, would be unpredictable and likely disappointing. Similarly, chemical reactions follow strict guidelines, and balancing chemical equations is like having a recipe for these reactions. It ensures that the number of atoms of each element on the reactant side (the ingredients) equals the number of atoms of the same element on the product side (the finished dish). This article will delve into the world of balancing chemical equations, focusing on worksheet 1 as a stepping stone for mastering this essential skill.
Understanding the Basics of Balancing Equations
Balancing chemical equations is essentially about conserving mass. It’s a fundamental principle in chemistry: atoms can’t be created or destroyed during a chemical reaction. They merely rearrange themselves to form new molecules. Therefore, the total mass of the reactants must equal the total mass of the products. This is where balancing comes in: ensuring that every element appears the same number of times on both sides of the equation.
The Art of Balancing: A Step-by-Step Guide
Balancing chemical equations involves adjusting coefficients, which are the numbers placed in front of each chemical formula. These coefficients represent the number of molecules involved in the reaction. Here’s a step-by-step process that can be used to balance chemical equations:
- Write the unbalanced chemical equation: Start with the chemical formulas of the reactants and products, separated by an arrow. For example: H2 + O2 -> H2O.
- Count the atoms of each element on both sides: In our example, observe that the reactants have 2 H atoms and 2 O atoms, while the product has only 2 H atoms and 1 O atom.
- Adjust coefficients to balance the atoms: Our goal is to make the number of atoms of each element identical on both sides. For this equation, we can start by placing a coefficient of 2 in front of H2O: H2 + O2 -> 2H2O. Now, the product side has 4 H atoms and 2 O atoms.
- Balance hydrogen atoms: To balance the hydrogen atoms, we need a coefficient of 2 in front of H2: 2H2 + O2 -> 2H2O.
- Check for balance: We now have 4 H atoms and 2 O atoms on both sides of the equation. The chemical equation is balanced!
Balancing Chemical Equations Worksheet 1: A Practice Ground
Worksheet 1 typically provides a series of unbalanced chemical equations that require students to apply the balancing principles. The worksheet may cover different types of reactions, such as:
- Synthesis reactions: Two or more substances combine to form a single product (e.g., Na + Cl2 -> NaCl).
- Decomposition reactions: A single compound breaks down into two or more simpler substances (e.g., CaCO3 -> CaO + CO2).
- Single replacement reactions: One element replaces another in a compound (e.g., Fe + CuSO4 -> FeSO4 + Cu).
- Double replacement reactions: The positive and negative ions of two reactants switch places (e.g., AgNO3 + NaCl -> AgCl + NaNO3).

Image: myans.bhantedhammika.net
Balancing Chemical Equations Worksheet 1 Answer Key: Solutions to Mastery
The answer key accompanying Worksheet 1 can serve as a guide for students to verify their balancing work. It’s important, however, to use the answer key strategically. Here are some tips:
- First attempt balancing independently: Don’t rely on the answer key immediately. Attempting to balance the equations yourself is the best way to learn and understand the process.
- Use the answer key as a reference: If you struggle with balancing a particular equation, check the answer key to see how it’s been done. Pay close attention to the coefficients and the steps involved.
- Analyze the differences: Compare your attempt with the answer key. Identify the areas where you went wrong and learn from your mistakes. This is how you truly improve your understanding.
Beyond Balancing: Applying the Knowledge
Balancing chemical equations is more than just a mathematical exercise; it’s a crucial step in understanding various chemical concepts. Here are some applications of balancing equations:
- Stoichiometry: This branch of chemistry deals with the quantitative relationships between reactants and products in chemical reactions. Balancing equations is essential for accurate calculations in stoichiometry, allowing scientists to predict the amounts of products formed or reactants needed for a specific reaction.
- Understanding chemical reactivity: Balancing equations helps us interpret how different chemical substances interact with each other. It allows us to see the exact ratios of reactants and products involved in a reaction, providing insights into the process itself.
- Developing new technologies: Balancing equations is fundamental to the development of new materials, pharmaceuticals, and energy technologies. By understanding the reactions involved, scientists can control the processes and optimize them for desired outcomes.
The Future of Balancing Equations: The Digital Frontier
Technology is rapidly changing the landscape of chemistry, including the way we balance equations. Online tools and software are being developed to assist students and scientists in this essential task. These platforms often provide:
- Automatic balancing: Enter the unbalanced equation, and the software will balance it for you, displaying the steps involved. This can be helpful for checking your work or exploring complex equations.
- Interactive tutorials: Many online tools offer interactive tutorials and practice exercises that allow students to learn and practice balancing equations in a dynamic and engaging way.
- Visualization and simulations: Some software allows you to visualize the chemical reactions and see how atoms rearrange themselves during the process, enhancing understanding and making the learning experience more intuitive.
Balancing Chemical Equations Worksheet 1 Answer Key
Conclusion: Mastering the Fundamentals
Balancing chemical equations, though seemingly straightforward, forms the foundation of understanding how chemical reactions occur and what products will be created. Worksheet 1, while a simple introduction, lays the groundwork for mastering this essential skill. Remember to practice, analyze your work, and use the answer key as a tool, not a crutch. As you become proficient in balancing equations, you’ll be taking a giant leap in your understanding of chemistry and its vast, exciting world. Now, go forth, balance those equations, and unlock the secrets of the chemical universe!





