Ever wondered why some elements are shiny and others dull, why some are highly reactive and others inert? The periodic table holds the answers, but unlocking its secrets requires understanding the trends that weave through its rows and columns. This is where graphing periodic trends comes in—a powerful tool that transforms abstract concepts into visual representations, making the periodic table come alive.
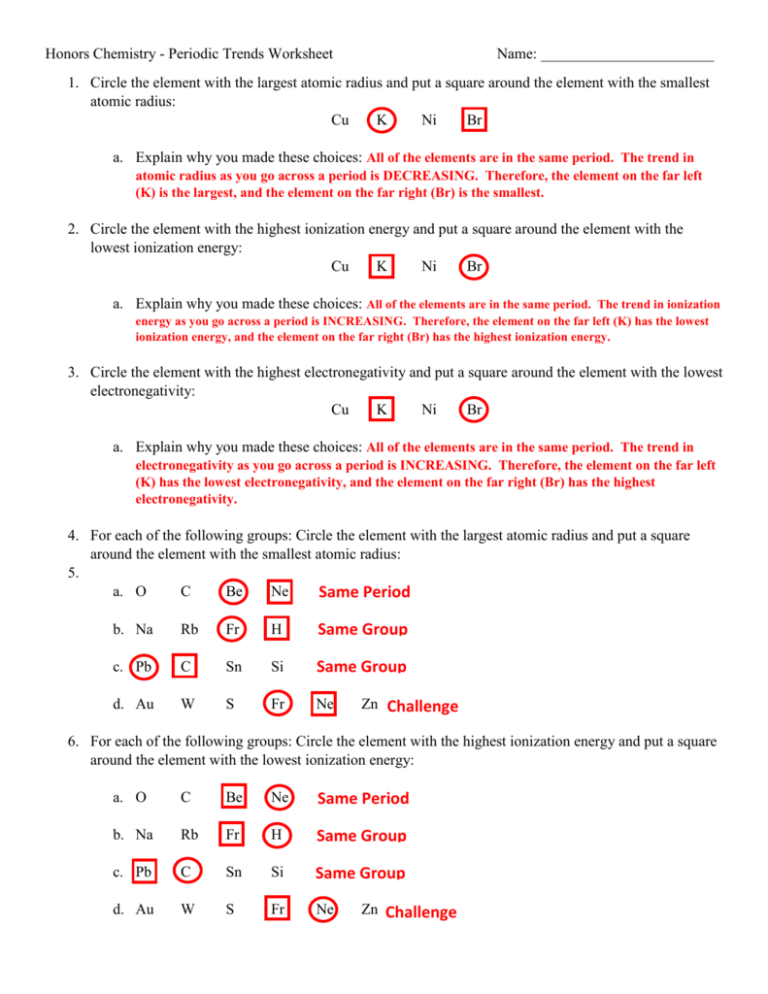
Image: davida.davivienda.com
Imagine a world where you could predict the properties of an element just by knowing its position on the table. This is the power of graphing periodic trends. Not only does it enhance your understanding of atomic structure and bonding, but it also helps you visualize the relationships between elements, leading to deeper insights and predictions.
Delving Deeper: The Essence of Periodic Trends
At the heart of the periodic table lies the fundamental concept of atomic structure: the arrangement of protons, neutrons, and electrons within an atom. This arrangement dictates an element’s chemical behavior and its place within the periodic table.
Electronegativity: The Tug of War for Electrons
One key trend to consider is electronegativity, which measures an atom’s tendency to attract electrons in a bond. Elements on the right side of the periodic table, closer to the halogens, have a stronger pull on electrons due to their smaller atomic radii and higher nuclear charge. Moving down a group, electronegativity generally decreases as the distance between the nucleus and valence electrons increases.
Ionization Energy: The Energy Required to Strip an Electron
Another important trend is ionization energy, the minimum energy needed to remove an electron from an atom in its gaseous state. As you move across a period, ionization energy generally increases because the increasing nuclear charge pulls the electrons more tightly. However, moving down a group, ionization energy decreases due to the greater distance between the nucleus and valence electrons.
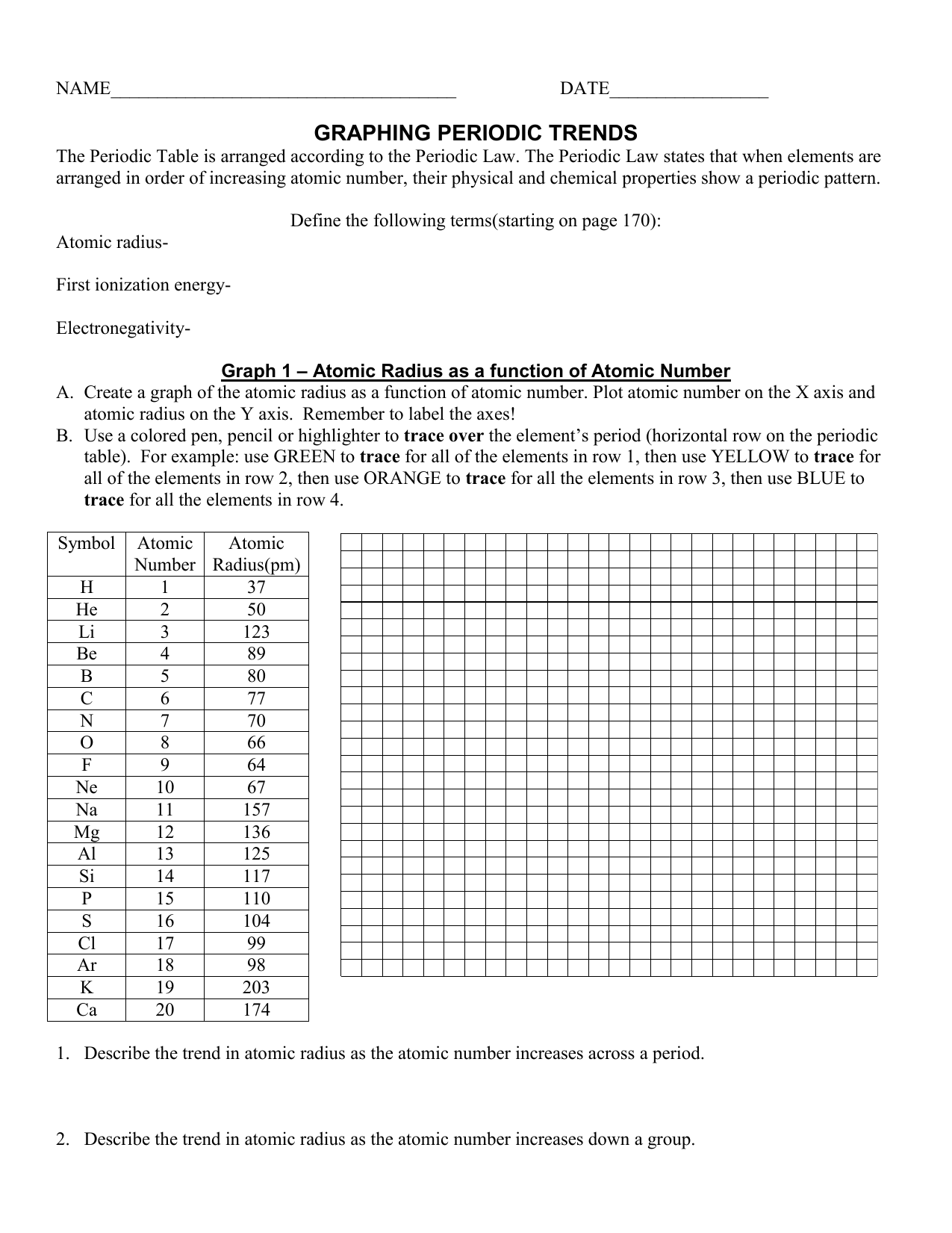
Image: imsyaf.com
Atomic Radius: Sizing Up the Atom
Atomic radius, the distance from the nucleus to the outermost electron shell, also exhibits predictable trends. Moving across a period, atomic radius decreases because the increasing nuclear charge pulls the electrons closer to the nucleus. Moving down a group, atomic radius increases as you add electron shells, pushing the outermost electrons further away from the nucleus.
Graphing Periodic Trends: Bridging the Gap between Theory and Reality
Now, let’s see how graphing these trends can propel your understanding.
Visualizing Trends: From Data Points to Insightful Graphs
Imagine plotting electronegativity values against atomic number. As you connect the points, you’ll notice a clear pattern: electronegativity generally increases as you move across a period and decreases as you move down a group. This visual representation transforms abstract data into a powerful tool for understanding and making predictions.
Similarly, graphing ionization energy against atomic number reveals a distinct upward trend across a period, reflecting the increasing nuclear charge and stronger hold on electrons. Moving down a group, however, the graph takes a downward turn, indicating the decreasing ionization energy due to the larger atomic radius.
Predicting Properties: The Power of Graphing
By creating graphs of periodic trends, you can predict the properties of elements based on their position within the periodic table. For instance, if you know the electronegativity of chlorine (Cl) is 3.16, you can estimate the electronegativity of bromine (Br), which sits below chlorine in the same group, to be slightly lower.
Beyond the Basics: Unveiling More Complex Relationships
Graphing periodic trends provides a framework for understanding not only simple trends but also complex relationships. For example, you can plot atomic radius against ionization energy for various elements. The resulting graph will reveal a negative correlation, where larger atomic radii generally correspond to lower ionization energies. This connection highlights the importance of visualizing the interconnectedness of different atomic properties.
Mastering Periodic Trends with Worksheet Answers and Beyond
Unlocking the secrets of the periodic table is a journey of exploration and discovery. Graphing periodic trends worksheets, accompanied by comprehensive answers, can be a valuable resource for your learning journey.
Guided Practice: Mastering the Fundamentals
These worksheets provide structured exercises that guide you through the process of plotting and interpreting periodic trends. The provided answers offer insights into the underlying principles and allow you to check your understanding.
Real-World Applications: The Importance of Periodic Trends
The concepts you learn through graphing periodic trends have far-reaching applications. For instance, predicting the reactivity of elements is crucial in chemical engineering, determining the properties of materials for different applications, and even understanding the formation of stars and galaxies!
Graphing Periodic Trends Worksheet Answers Pdf
Conclusion: Embark on Your Periodic Journey
Graphing periodic trends is not just about creating visual representations; it’s about developing a deeper understanding of the principles that govern the behavior of elements and their compounds. By visualizing and interpreting these trends, you can unlock new insights, make informed predictions, and gain a profound understanding of the periodic table. So, grab your periodic table, your graphing calculator, and embark on this fascinating journey of discovery!





