Have you ever stared at a chemistry worksheet filled with mole to mole ratios, feeling a wave of confusion wash over you? You’re not alone. Many students find this concept challenging, but it’s crucial for understanding chemical reactions and calculations. This article will break down the mysteries of mole to mole ratios, equipping you with the knowledge and skills to conquer any worksheet!

Image: www.worksheeto.com
Mole to mole ratios are essential for balancing chemical equations and predicting the amounts of reactants and products involved. Imagine baking a cake: you need specific quantities of flour, sugar, eggs, and other ingredients for it to turn out perfectly. In chemistry, these “ingredients” are chemical substances, and mole to mole ratios tell us the precise amounts needed to create a successful reaction.
Understanding the Foundation: Mole Concept and Balanced Equations
The Mole: Chemistry’s Unit of Measurement
The mole is the cornerstone of chemistry, serving as the standard unit for measuring the amount of a substance. One mole contains 6.022 x 1023 particles, be they atoms, molecules, or ions. This colossal number, better known as Avogadro’s number, provides a convenient way to quantify the vast quantities of particles involved in chemical reactions.
Balancing Chemical Equations: A Symphony of Moles
Chemical equations are like recipes, outlining the reactants (ingredients) and products (final dish) of a reaction. Balancing these equations ensures that the number of atoms of each element on both sides of the equation is equal, reflecting the law of conservation of mass. Mole to mole ratios arise from these balanced equations, showcasing the precise relationship between the different reactants and products.
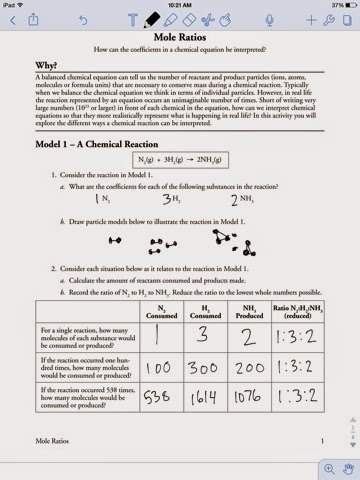
Image: www.semanarioangolense.net
Mastering Mole to Mole Ratios: A Step-by-Step Guide
Let’s delve into the practical side of mole to mole ratios. Here’s a step-by-step guide to tackling those tricky worksheet questions:
Step 1: Writing the Balanced Chemical Equation
Before you can even think about mole to mole ratios, you must have a balanced chemical equation. Let’s take the classic example of the reaction between hydrogen gas and oxygen gas to form water:
H2 (g) + O2 (g) → H2O (l)
This equation isn’t balanced because there are two oxygen atoms on the left side and only one on the right. To balance it, we add a coefficient of 2 in front of H2O:
2H2 (g) + O2 (g) → 2H2O (l)
Now, the equation is balanced – we have 4 hydrogen atoms and 2 oxygen atoms on both sides.
Step 2: Identifying the Mole Ratio
The coefficients in a balanced equation represent the mole to mole ratio between the substances. In our water formation example, the coefficients are 2, 1, and 2. This means:
- 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water.
Step 3: Solving the Problem
Now, let’s consider a problem: How many moles of water can be produced from 4 moles of hydrogen gas, given the balanced equation:
2H2 (g) + O2 (g) → 2H2O (l)
Here’s how to solve it:
- 1. **Identify the mole ratio:** 2 moles of H2 : 2 moles of H2O
- 2. **Set up a proportion:** (2 moles H2) / (2 moles H2O) = (4 moles H2) / (x moles H2O)
- 3. **Solve for x:** x = 4 moles H2O
Therefore, 4 moles of hydrogen gas will produce 4 moles of water.
The Beauty and Importance of Mole to Mole Ratios
Mole to mole ratios are more than just calculations on a worksheet. They are the backbone of stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
Predicting Reactions and Quantifying Products
Mole to mole ratios allow us to predict the outcome of chemical reactions. They tell us how much of a reactant is needed to completely react with another, and how much product will be formed. In the world of industrial chemistry, this is crucial for optimizing production processes and minimizing waste.
Understanding Chemical Reactions at a Deeper Level
Understanding mole to mole ratios elevates your understanding of chemical reactions. It transforms you from a passive observer to an active participant, enabling you to predict and manipulate chemical processes.
Real-World Applications: From Everyday Life to Industry
Mole to mole ratios are not confined to the laboratory. They are essential in various fields, touching our lives in countless ways:
Food Production and Nutrition
Chemical reactions are essential for food production. From baking bread to brewing beer, mole to mole ratios ensure the proper proportions of ingredients for optimal results. Even in nutrition, these ratios help us understand how our bodies utilize different nutrients.
Environmental Science and Climate Change
Understanding mole to mole ratios is crucial for tackling environmental challenges. By analyzing the ratios of reactants and products in pollution-generating reactions, we can devise strategies to reduce emissions and mitigate climate change.
Pharmaceutical Development and Medicine
In pharmaceutical research and development, mole to mole ratios are fundamental for producing drugs and understanding their effects on the human body. The accurate synthesis of medications relies heavily on these ratios.
Boosting Your Confidence: Mastering Mole to Mole Ratios
The key to mastering mole to mole ratios is practice. Work through various problems, starting with simple ones and gradually increasing difficulty. Don’t be afraid to ask for help from teachers, tutors, or online resources. With dedicated effort, you’ll develop a confident grasp of this essential chemical concept.
Mole To Mole Ratio Worksheet Answers
Conclusion
Mole to mole ratios are a fundamental concept in chemistry with profound implications for understanding and manipulating chemical processes. Whether you’re a student tackling homework assignments or a professional working in a chemistry-related field, a thorough grasp of this topic is essential. So, keep practicing your skills, and you’ll be well on your way to becoming a mole-to-mole ratio master!





