Have you ever wondered how cooking transforms raw ingredients into delicious meals? Or how rust forms on your bicycle? These are just a glimpse into the fascinating world of chemical reactions, the building blocks of everything around us. Chemistry, at its core, is the study of matter and its transformations, and chemical reactions are the processes that drive these changes. But studying these transformations can be challenging, especially when you’re first introduced to the concept. That’s where a “types of chemical reactions worksheet with answers” comes in handy. It can serve as a guide, helping you understand the different types of chemical reactions while providing opportunities to practice and test your knowledge.

Image: www.chemistrylearner.com
This worksheet is not just about memorizing definitions; it’s about developing a deeper understanding of how these reactions work and how they apply to our world. By working through the exercises and analyzing the answers, you’ll gain insight into the fundamental principles of chemistry and discover how these reactions influence our daily lives, from the food we eat to the energy we use. Let’s embark on a journey through the world of chemical reactions, exploring the four main types and their applications using a worksheet as our guide.
Understanding the Basics: What are Chemical Reactions?
Imagine a puzzle where pieces constantly rearrange themselves, creating new patterns and combinations. Chemical reactions are like this, except they involve atoms and molecules instead of puzzle pieces. In chemical reactions, atoms and molecules break apart, rearrange, and recombine to form new substances with different properties. Think of baking a cake: mixing flour, sugar, eggs, and butter results in a new substance entirely different from the ingredients themselves. This transformation is a chemical reaction.
Types of Chemical Reactions: A Categorical Guide
Chemical reactions are diverse, but they can be categorized into four main types: synthesis, decomposition, single displacement, and double displacement. Each type is characterized by distinct patterns of rearrangement and products formed.
1. Synthesis Reactions: Building Blocks of Chemistry
Imagine Legos, each piece representing an atom or molecule. In a synthesis reaction, you’re essentially assembling those Legos, combining two or more reactants to form a single, more complex product. The general format looks like this: A + B → AB. For example, when iron reacts with oxygen, it produces iron oxide, commonly known as rust: 4Fe + 3O2 → 2Fe2O3. Think of this as the foundation of chemical reactions, laying the groundwork for creating new materials and compounds.
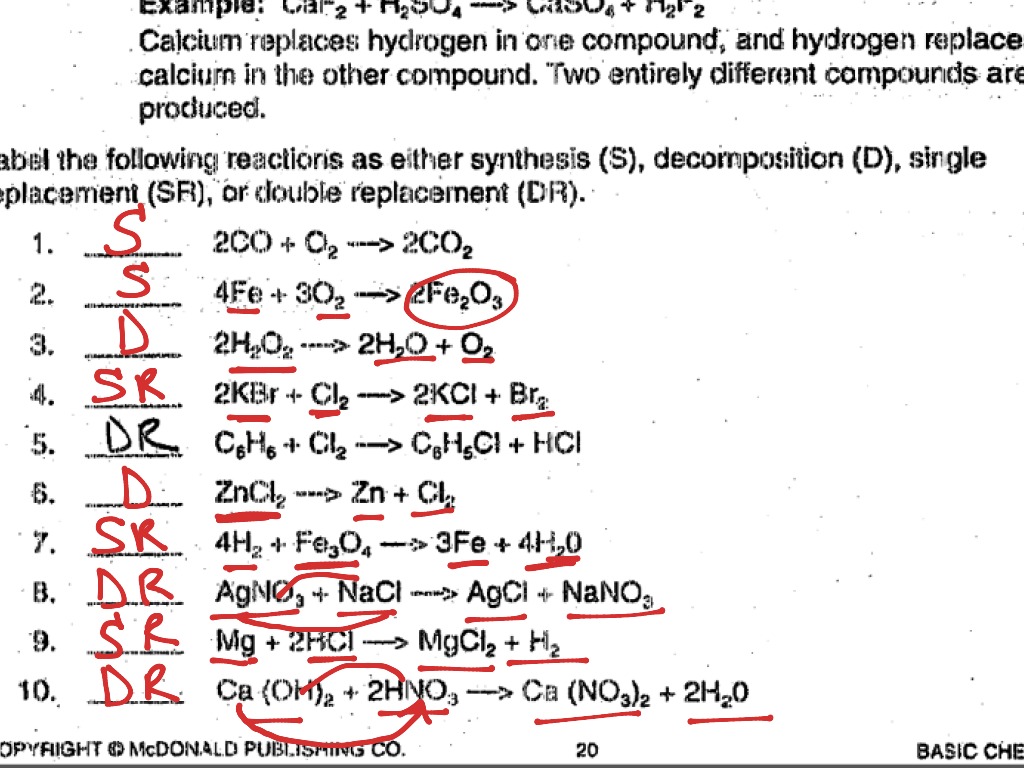
Image: wordworksheet.com
2. Decomposition Reactions: Breaking Down Complex Molecules
Now, let’s reverse the process. Decomposition reactions are like taking apart those Legos, breaking down a single, complex reactant into two or more simpler products. The general format is: AB → A + B. A classic example is the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2): 2H2O2 → 2H2O + O2. These reactions are essential for breaking down complex molecules, like food digestion, and even in the production of certain chemicals.
3. Single Displacement Reactions: Switching Partners
In this type of reaction, one element swaps places with another element in a compound, like two friends switching dance partners. The general format is: A + BC → AC + B. For instance, when zinc (Zn) reacts with hydrochloric acid (HCl), zinc displaces hydrogen (H) to form zinc chloride (ZnCl2) and hydrogen gas (H2) is released: Zn + 2HCl → ZnCl2 + H2. Think of this type of reaction as a “swap,” where a more reactive element replaces a less reactive one.
4. Double Displacement Reactions: A Partner Swap
Imagine a dance where all the partners switch! That’s essentially what happens in a double displacement reaction. Two compounds exchange their cations (positively charged ions) and anions (negatively charged ions) to form two new compounds. The general form is: AB + CD → AD + CB. For example, when silver nitrate (AgNO3) reacts with sodium chloride (NaCl), a white precipitate, silver chloride (AgCl), forms: AgNO3 + NaCl → AgCl + NaNO3. These reactions are especially relevant in precipitation reactions, where an insoluble solid separates from the solution.
Types of Chemical Reactions Worksheet with Answers: Your Guide to Mastery
Now that we’ve explored the types of chemical reactions, let’s dive into the worksheet and put this knowledge into practice:
-
Section 1: Identifying the Type of Reaction: This section presents a series of chemical equations. Using the knowledge we’ve gained, you’ll identify the type of reaction based on the pattern of rearrangements. Examples include:
- CaO + CO2 → CaCO3 (Identify the reaction type: Synthesis)
- 2KClO3 → 2KCl + 3O2 (Identify the reaction type: Decomposition)
- Cu + 2AgNO3 → Cu(NO3)2 + 2Ag (Identify the reaction type: Single Displacement)
- BaCl2 + Na2SO4 → BaSO4 + 2NaCl (Identify the reaction type: Double Displacement)
-
Section 2: Writing Chemical Equations: In this section, you will be provided with descriptions of chemical reactions, and you’ll need to write the balanced chemical equation based on the given information. For example:
- “Sodium reacts with chlorine gas to produce sodium chloride.” (Write the balanced equation: 2Na + Cl2 → 2NaCl)
- “Calcium carbonate decomposes into calcium oxide and carbon dioxide.” (Write the balanced equation: CaCO3 → CaO + CO2)
-
Section 3: Applying Chemical Reactions in Everyday Life: This section focuses on applying the knowledge of chemical reactions to real-world scenarios. For instance, you might be asked about:
- “What type of reaction is involved in the rusting of iron?” (Answer: Synthesis)
- “How is baking soda (NaHCO3) used in baking?” (Answer: Sodium bicarbonate decomposes when heated, releasing carbon dioxide gas, which causes the batter to rise)
Types Of Chemical Reactions Worksheet With Answers
Unlocking the Power of Chemical Reactions: Your Journey Continues
By completing the types of chemical reactions worksheet with answers, you’ve gained a deeper understanding of the building blocks of the world around you. These reactions, from the simple to the complex, are the driving force behind everything, from the formation of stars and planets to the life processes within our bodies. It’s a journey that continues as you explore more advanced concepts in chemistry, building upon the foundation laid by this worksheet. Remember, learning is an ongoing process, and the more you explore, the more you’ll unravel the mysteries of the chemical world and discover the wonders hidden within.





