Ever wondered how the solid ice in your freezer can transform into liquid water, then vaporize into the air? The answer lies in the fascinating world of states of matter, the various forms that matter can take. Understanding these states and their transformations is crucial for grasping essential scientific principles that govern our everyday lives. In this comprehensive guide, we’ll explore the states of matter through the lens of a simulation lab, providing you with an answer key to unlock your knowledge and unlock the mysteries of the physical world.

Image: marlongokegarza.blogspot.com
Whether you’re a student embarking on your scientific journey or a curious mind wanting to delve deeper, this lab will serve as your virtual playground for exploring the properties and behaviors of matter. We’ll cover the fundamental states of matter, delve into their unique characteristics, and analyze the factors that govern transitions between states. By interacting with interactive simulations, you’ll gain hands-on experience that transforms theoretical knowledge into tangible understanding.
Unveiling the States of Matter
Before we dive into the lab, let’s first define the basic states of matter that we’ll be exploring: solid, liquid, gas, and plasma. Each state exhibits distinct properties due to the arrangement and motion of its constituent particles – atoms and molecules. Let’s examine each state in detail:
Solids: Rigid and Well-Ordered
Imagine building a block tower using Lego bricks. Each brick represents an atom or molecule in a solid. These particles are tightly packed together in a fixed, repeating arrangement, creating a rigid structure. As a result, solids have a definite shape and volume. Think of the sturdy table you’re sitting at or the icy cube in your glass; these are examples of solids. Solids hold their shape because their particles have very limited freedom of motion; they can only vibrate about their fixed positions.
Liquids: Flowing and Adaptable
Now imagine pouring water into a glass. The water takes the shape of the glass but maintains its volume. That’s because the particles in a liquid are closer together than those in a gas, but not as tightly bound as in a solid. They have more freedom to move and slide past each other, allowing liquids to flow and take the shape of their container. Liquids are nearly incompressible, meaning their volume remains fairly constant regardless of pressure. Think of pouring a glass of juice or watching the rain fall; these are everyday examples of liquids.
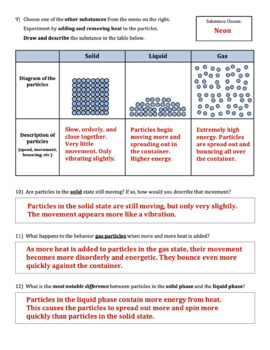
Image: trendingonsocialmediathisweek389.blogspot.com
Gases: Free and Energetic
Imagine a balloon filled with air. The air inside can expand to fill the entire balloon, taking on its shape. That’s because particles in a gas have the most freedom of motion. They are widely spaced and move randomly at high speeds, colliding with each other and with the walls of their container. Gases are highly compressible, meaning their volume can be easily reduced by increasing the pressure. Imagine cooking with gas, or observing steam rising from a boiling pot of water; these are familiar examples of gases.
Plasma: The Energetic State
While less familiar, plasma is a state of matter often referred to as the “fourth state.” This highly energized state exists when atoms lose electrons, forming an ionized gas consisting of free ions and electrons. Plasma, though similar to gases, exhibits unique properties due to its electrical conductivity, and it’s often associated with high temperatures. The aurora borealis, or Northern Lights, is a beautiful example of plasma in the Earth’s atmosphere, while fluorescent lights and neon signs are other examples showcasing plasma application.
Navigating the States of Matter Simulation Lab: Your Answer Key
Now, let’s embark on a virtual journey through our simulation lab to explore the states of matter firsthand. Prepare to tinker with variables, observe transformations, and answer key questions that will solidify your understanding.
Experiment 1: Thermal Energy and State Changes
Our first experiment focuses on the key concept of thermal energy, also called heat. Heat is a form of energy that affects the motion of particles within a substance. As we add heat, particles move faster.
In the simulation, you’ll see a container filled with a substance. You can control the temperature, viewing the substance as it changes states. For example, you might observe ice melting into water, or water boiling into steam. Analyze the simulation and answer the following questions:
- What happens to the particles as the temperature increases? Answer: As temperature increases, particles vibrate more rapidly and their average kinetic energy increases. This is illustrated by the increased movement of particles within the simulation.
- How does the spacing between particles change between the states? Answer: As a substance changes from solid to liquid to gas, the particles become more widely spaced. The simulation allows you to visually observe this difference.
- What is the role of thermal energy in state changes? Answer: Thermal energy provides the energy required to overcome the forces holding particles together. For example, as you heat a solid, particles gain enough energy to break free from their fixed positions and move more freely as a liquid.
Experiment 2: Pressure and State Changes
Our next experiment explores the concept of pressure. Pressure refers to the force exerted by a substance on a given area. In gases, pressure increases as molecules collide more frequently with the walls of their container.
The simulation allows you to observe the effect of pressure on the state of a substance. You may see pressure changes cause a substance to transition from gas to liquid, or from liquid to solid. Answer these questions:
- How does pressure affect the volume of a gas? Answer: As pressure on a gas increases, the volume decreases. Think of a syringe with air inside – pressing down the plunger decreases the volume.
- Can pressure be used to change the state of matter? Answer: Yes, pressure is a key factor in state changes. For example, pressure can cause a gas to liquefy (become a liquid).
- Describe how pressure and temperature relate when considering state changes. Answer: Both pressure and temperature play significant roles in state changes. Increasing pressure tends to favor a denser state, while increasing temperature tends to favor a less dense state.
Experiment 3: The Role of Intermolecular Forces
Our final experiment focuses on the role of intermolecular forces, which are the attractive forces between molecules. These forces vary in strength and significantly influence the properties of different states of matter.
The simulation allows you to compare how the intermolecular forces differ between substances. For example, you may observe that water has stronger intermolecular forces than a substance like argon. Answer these key questions:
- How do the intermolecular forces affect the boiling point of a substance? Answer: Substances with stronger intermolecular forces have higher boiling points. This is because more energy is required to overcome these attractions and separate the molecules into a gaseous state.
- How do intermolecular forces explain the different states of matter? Answer: Intermolecular forces are directly responsible for the differences between the states. The strength of these forces determines how closely packed the particles will be, and how much they will be able to move around.
- Name some substances with strong and weak intermolecular forces, and describe their properties. Answer: Water has relatively strong intermolecular forces, leading to its high boiling point and liquid state at room temperature. Argon, on the other hand, has weak intermolecular forces, resulting in it being a gas at room temperature.
Beyond the Lab: Real-World Applications
The concepts we’ve explored in this lab have far-reaching applications in our daily lives and scientific endeavors:
- Weather Forecasting: Understanding the states of matter is essential for weather forecasting. Meteorologists use data on temperature, pressure, and humidity to predict precipitation, cloud formation, and other weather phenomena.
- Medicine and Biology: States of matter play a crucial role in medicine and biological processes. For example, the freezing and thawing of cells, the vaporization of anesthesia, and the diffusion of gases in the lungs all rely on principles related to states of matter.
- Materials Science and Engineering: By understanding the properties of different states of matter, scientists and engineers develop new materials with specific properties. This includes designing stronger and lighter alloys, synthesizing advanced polymers, and creating superconductors.
States Of Matter Simulation Lab Answer Key
Conclusion
The world of states of matter is a fascinating and dynamic one. By mastering the concepts we’ve explored in this virtual lab, you’ve gained a deeper understanding of the fundamental principles that govern the behavior of matter. This knowledge empowers you to appreciate the complexity of our physical world and to utilize these principles in various fields. So, delve deeper into the world of states of matter, explore further readings, and share your newfound knowledge with those around you. The journey of scientific discovery knows no bounds.





